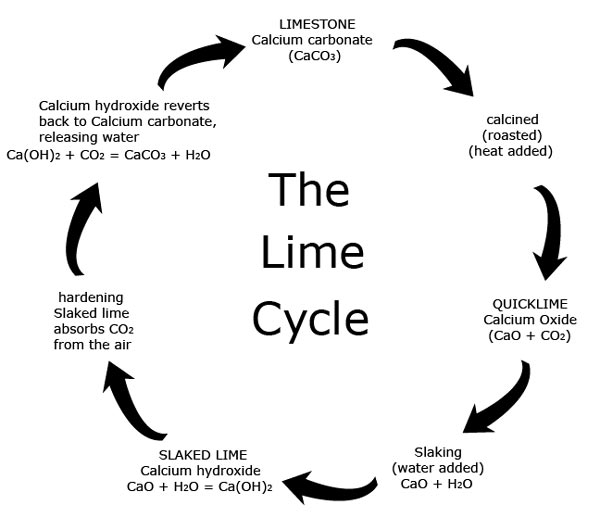
When limestone is affected by heat or acid it transforms into a series of unstable chemical compounds, before eventually turning back into Calcium carbonate. This process is known as the 'Lime Cycle'.
When limestone (calcium carbonate) (CaCO3) is heated Carbon dioxide (CO2) is driven off leaving calcium oxide (CaO) which is also known as quicklime.
CaCO3 = CaO + CO2
Quicklime is chemically unstable and caustic.
When quicklime is mixed with water (H2O) (a process called hydration or slaking) heat and steam are released and the quicklime turns into calcium hydroxide Ca(OH)2. Calcium hydroxide is also known as slaked lime.
CaO + H2O = Ca(OH)2
The slaked lime reacts with carbon dioxide (CO2) in the air, slowly releasing water and hardening as it reverts back to calcium carbonate.
Ca(OH)2 + CO2 = CaCO3 +H2O
This process can occur in nature (have a look at the geology pages), but can also be easily made to happen by people.
Alkalis
An alkali is a 'salt' of a metal (in this case Calcium) dissolved in water. It is the opposite of an acid. Alkalis have a pH greater than seven, acids have a pH less than seven. Both acids and alkalis can burn your skin.
